Alliance for Hippocratic Medicine v. U.S. Food and Drug Administration
Update: This evening, the U.S. Supreme Court granted a stay of the lower court’s orders to constrain the distribution of abortion drugs while the case continues on appeal at the Fifth Circuit. The Court as a whole decided to allow the drug to continue for now. Concerned Women for America (CWA) CEO & President Penny Nance criticized the ruling saying, “The law is clear, so we are confident of our ultimate victory. The FDA’s reckless disregard for women’s safety in the rushed approval of the abortion drug for political purposes will be exposed. But we are deeply saddened to see the Court ignore the untold number of women who will be at risk because the FDA decided to lower the standards of care to promote abortion to an ever-increasing number of women.” Justice Clarence Thomas and Samuel Alito dissented both saying they would have denied the stay. Justice Thomas did not explain his reasoning further, but Justice Alito said plainly, “At present, the applicants are not entitled to a stay because they have not shown that they are likely to suffer irreparable harm in the interim.” The case will continue in the Fifth Circuit where we hope the stay will once again be issued, which will virtually guarantee the U.S. Supreme Court will get an appeal once again. The court is scheduled to consider the matter on May 17, 2023.
Yesterday, the United States Supreme Court extended its previous order halting until this Friday the Fifth Circuit Court of Appeals stay, limiting the distribution of the abortion drug while the case challenging its original approval continues. This means the life-ending drug is still available in an expanded fashion (even through the mail), as the Biden Food and Drug Administration (FDA) and Department of Justice (DOJ) have been promoting using the COVID-19 emergency as their excuse.
The move suggests the Court is taking the matter seriously and needs more time before rendering a decision. Check back here on Friday for the latest developments on this important fight. We pray this added time gives the Court time to study the corrupted history of approving this unsafe drug more carefully.
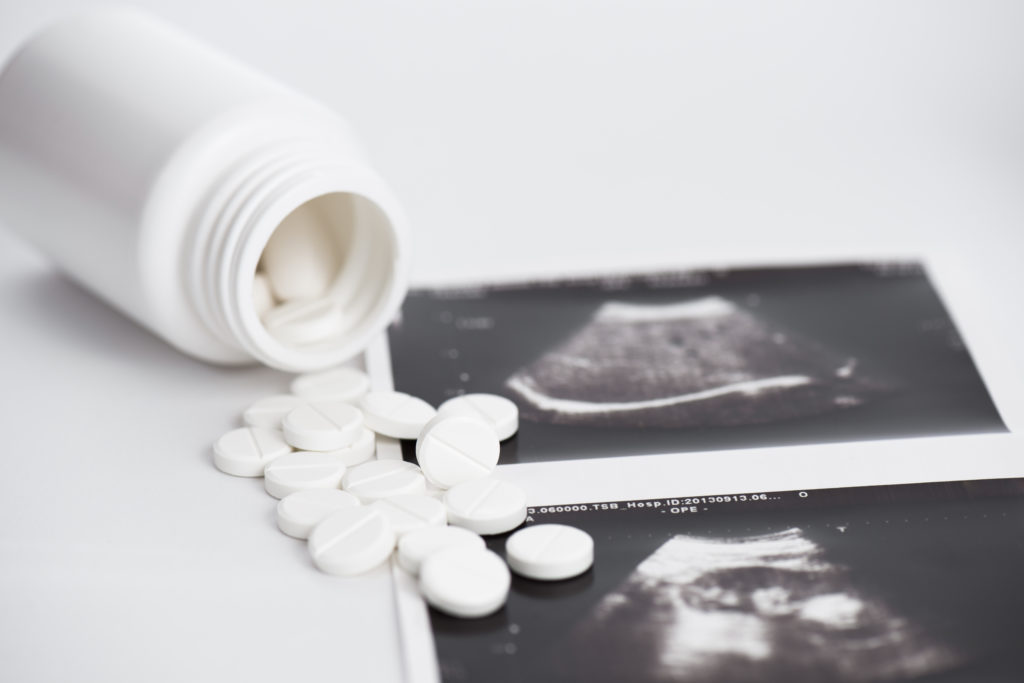
The FDA approved the pill under its accelerated approval program, created in the early 1990s, to speed access to the first HIV drugs. It has been used to expedite drugs for cancer and other “serious or life-threatening diseases.” But pregnancy is not a disease. Still, the FDA chose to look at it as such to accomplish its political goals.
Beyond the initial approval, the FDA has removed the most basic standards of care for women’s safety. They increased the gestational age for which a pregnant woman can take chemical abortion drugs; they changed the dosage, significantly reduced the number of required in-person visits, and even allowed non-doctors to prescribe and administer chemical abortions, in addition to other changes. More recently, they’re promoting the distribution of this dangerous drug through the mail in violation of federal law.
That is one of the reasons why the district court granted a stay to the FDA’s reckless approval, halting its distribution while the case moves forward. The district court simply noted the very text of the Comstock Act prohibits the mailing of chemical abortions, and DOJ cannot amend it with a simple judicial opinion.
Beyond that, the court concluded the FDA’s actions in approving this drug likely violated the Administrative Procedures Act as “arbitrary and capricious.” There are many reasons for this. In its original approval in 2000, the FDA initially proposed significant restrictions on the drugs to the manufacturer in private. Once the restrictions were leaked, the company rejected them, and political pressure mounted to the point that the FDA abandoned them completely, despite its “serious reservations” about mifepristone’s safety. The data supported the FDA’s concerns, but it ignored them for political, not scientific, reasons.
The FDA then doubled down on its reckless disregard for women’s safety in 2016 when it further loosened the safety requirements on the drugs based on little to no data. It used studies using the safety requirements they were abandoning to justify the same action of removing them.
Similarly, at this point, the FDA abandoned requirements for reporting non-fatal adverse events. This is a significant event because, in further loosening restrictions in 2021, it cited the lack of non-fatal adverse events as an indication that the drug is safe. Again, both the district and appellate courts noted this in restricting the FDA’s actions. The Fifth Circuit said, “This ostrich’s -head-in-the-sand approach is deeply troubling….” The district court put it this way, “[I]t is circular and self-serving to practically eliminate an ‘adverse event’ reporting requirement and then point to a low number of ‘adverse events’ as a justification for removing even more restrictions than were already omitted in 2000 and 2016.”
Both courts also noted as troubling the fact that this drug has never been tested for under-18 girls, while it is being increasingly made available to them as “safe.” Based on no evidence at all.
For these and many other reasons, it is not unreasonable to ask the FDA to do its job and ensure this drug is safe by following its regular protocols before it promotes it as safe for women to use regularly, without knowing the long-term effects on women’s health, especially in the case of young women.
The appellate court limited its stay to the expansions after the initial approval because of a statute of limitation that prevents the challenge of FDA procedures after six years, but the court will review whether, as the district court concluded, it was the FDA’s own actions that prevented timely review. Once again, the FDA wants to profit from the events it created by delaying the administrative challenge of the doctors who are suing to protect women’s safety in this case.
The doctors are being represented by the Alliance Defending Freedom (ADF). And Concerned Women for America has submitted a brief supporting their arguments on behalf of conservative women around the country.
Please continue to pray for justice in this case. Though the case is now before the U.S. Supreme Court, they will only review whether the drug will be available and to what extent while the case moves forward. Eventually, the full review of the FDA’s actions will be done by the district court, and challenges are sure to follow.
It will be a long process, but one well worth our involvement as we stand for life and women’s safety in a post-Roe world.